
The values of the α 2 H parameter, which represents the ability of a substituent to be donor of hydrogen bonds, show that the presence of fluorine atoms notably enhances the hydrogen bond donor ability of a hydroxyl group. Moreover, data acquisition of 1H-NMR is rapid enough to follow the tautomeric rearrangement in this system in real time, providing thermodynamic and kinetic parameters. 64 This should be particularly appreciated in view of the fact that these polymers do not crystallize.

Thus, the complex hydrogen bonding of the linker group is fully elucidated.
The homonuclear 1H- 1H 2D DQ NMR spectra differ markedly for the two forms, see Figure 29, making it possible to determine the 1H- 1H as well as the 1H- 15N distances (after moderate 15N-labeling) with high accuracy. In order to exploit fully the advantages offered by this approach, a detailed characterization of the hydrogen-bonded moieties in the solid state is essential. 63 These systems are sometimes called ‘smart materials’, as the hydrogen bonds can reversibly rearrange at elevated temperatures, which makes them suitable for use in hot melts and coatings, where a reversible, strongly temperature-dependent rheology is highly desirable. In fact, using bifunctional monomers with two ureido-pyrimidinone moieties inspired by nucleic acids linear supramolecular polymers held together by quadruple hydrogen bonds have been synthesized.
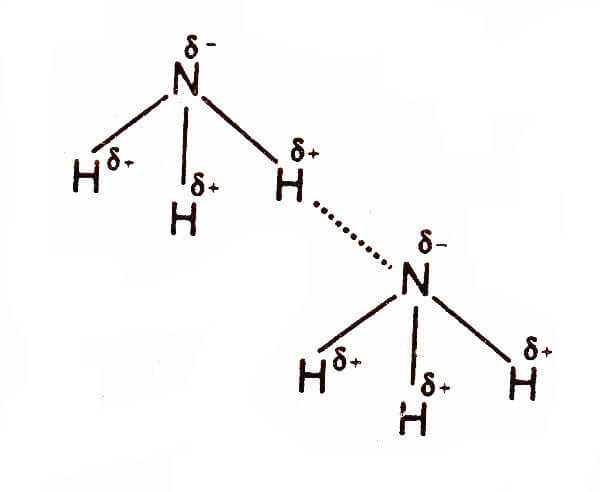
Hydrogen bonds provide probably the most important secondary interactions exploited in natural and supramolecular systems.


 0 kommentar(er)
0 kommentar(er)
